

A new multicentre study reveals why the structure of the ApoB100 protein, present in LDL together with so-called “bad cholesterol”, plays a crucial role in the propensity of LDL to accumulate in the arterial walls of patients with familial hypercholesterolaemia, thus promoting the formation of atherosclerotic plaque.
The work is co-led by the researcher from the Institute for Biomedical Research of Barcelona (IIBB-CSIC) and CIBERCV, Vicenta Llorente Cortes, and the researcher from the University Toulouse Paul Sabatier, Valerie Samouillan.
Familial hypercholesterolaemia is a relatively common genetic disorder. It affects approximately one in every 200 or 300 people. Those affected have had high levels of low-density cholesterol (LDL) since birth and, consequently, a higher risk of cardiovascular disease and increased rates of premature death from this cause.
Why do LDL particles clump together more in this congenital disease? Are there biochemical and physical differences that explain it? That is what they have tried to clarify in this study, published in the Journal of Lipid Research.
The work involved 10 research centres in Spain and France. Among them, and in addition to the IIBB-CSIC and CIBER, are the Institute of Materials Science of Barcelona (ICMAB-CSIC), the Sant Pau Research Institute (IR Sant Pau), the Autonomous University of Barcelona (UAB), the CIRIMAT Institute (Toulouse, France) and the Miguel Servet Hospital in Zaragoza.
LDL particles in patients with familial hypercholesterolaemia show a greater tendency to aggregate and form plaques. This is due, explains Vicenta Llorente Cortes, “to the fact that the ApoB100 protein in LDL has a particular structural conformation, with a high percentage of rigid alpha helices [secondary structures], compared to the LDL of healthy patients.”
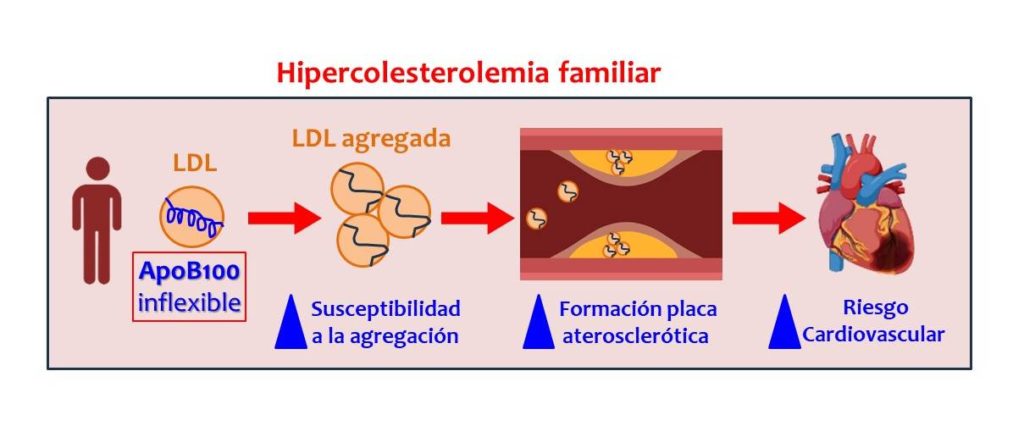
Based on samples from 35 patients with familial hypercholesterolaemia and 29 healthy individuals as a control group, the researchers have demonstrated that in patients with familial hypercholesterolaemia, the protein present in LDL is smaller due to its high esterified cholesterol content and has less structural flexibility compared to the LDL of healthy individuals. As a result, these LDL exhibit a reduced ability to recover their structure in the arterial intima, favouring their accumulation in the inner wall of the arteries.
Among other things, the study measured how easily LDL particles clump together using dynamic light scattering techniques, as well as the size, composition and structure of LDL particles by electron microscopy.
As Llorente explains, one of the most impactful findings was detecting the difference in the percentage of flexible secondary structures in the ApoB100 of patients with FH, which would not have been possible without the collaboration of the biophysics group led by Dr Samouillan (University of Toulouse). This group applied FTIR infrared spectroscopy to determine the structure of the protein and, in particular, to quantify the content of stable alpha helices and flexible alpha helices in the LDL of the control groups and patients.
The results suggest that developing strategies to structurally preserve ApoB100, and in particular the percentage of flexible alpha helices in LDL, could be a new way to reduce the risk of heart disease in these patients.
This finding provides a new perspective for understanding how alterations in the structure of ApoB100 can directly influence the risk of developing cardiovascular diseases. It also opens up possibilities for designing specific therapies aimed at modulating the flexible alpha helix content in LDL, helping to prevent atherosclerosis.
“In our research group,” adds Vicenta Llorente, “we are comparing whether PCSK9 inhibitors [a type of drug] can help preserve the percentage of flexible alpha helices and whether these effects are comparable with those achieved through innovative peptide tools developed in our group specifically for this purpose. With these new peptide tools, we aim to preserve the structural flexibility of the ApoB100 protein in the LDL of patients with familial hypercholesterolaemia.”
Maria Teresa La Chica Lhoëst, Andrea Martínez, Eduardo Garcia, Jany Dandurand, Anna Polishchuk, Aleyda Benitez-Amaro, Ana Cenarro, Fernando Civeira, Amable Bernabé, David Vilades, Joan Carles Escolà-Gil, Valerie Samouillan, Vicenta Llorente-Cortes. ApoB100 remodeling and stiffened cholesteryl ester core raise LDL aggregation in familial hypercholesterolemia patients. Journal of Lipid Research. https://pubmed.ncbi.nlm.nih.gov/39557294/